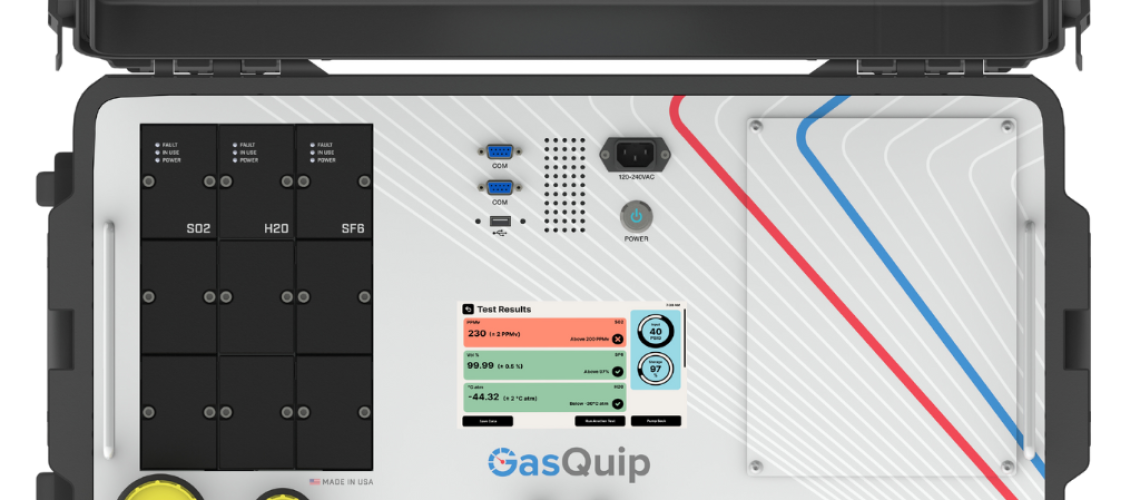
Interpreting the results of an SF6 analyzer measurement can be a bit daunting, especially if you are not familiar with the various parameters that are measured and the units in which they are reported. In this blog post, we will discuss some of the key parameters measured by an SF6 analyzer and how to interpret the results.
The 3 Standard Gases
- SF6 concentration: The most fundamental parameter measured by an SF6 analyzer is the concentration of SF6 gas in the sample. This is typically reported volume percent (%). A typical concentration range for SF6 gas in electrical equipment is between 98-99.9%.
- Moisture: The presence of moisture in SF6 gas can adversely affect its performance as an insulator. Moisture can also cause corrosion of electrical equipment. An SF6 analyzer may measure the moisture content in the gas sample in parts per million by volume (ppmv) or dew point temperature. Dew point temperature is the temperature at which moisture in a gas condenses into liquid form.
- Sulfur Dioxide: When an SF6 circuit breaker has high levels of sulfur dioxide measured in the gas, it suggests that there is decomposition of SF6 due to overheating or arcing. The decomposition of SF6 produces sulfur dioxide and other toxic byproducts, which can damage the equipment and pose health risks to those exposed to the gas. It is important to promptly identify and address the cause of the decomposition to prevent further damage and ensure the safe operation of the circuit breaker. Typically measured in (ppmv)
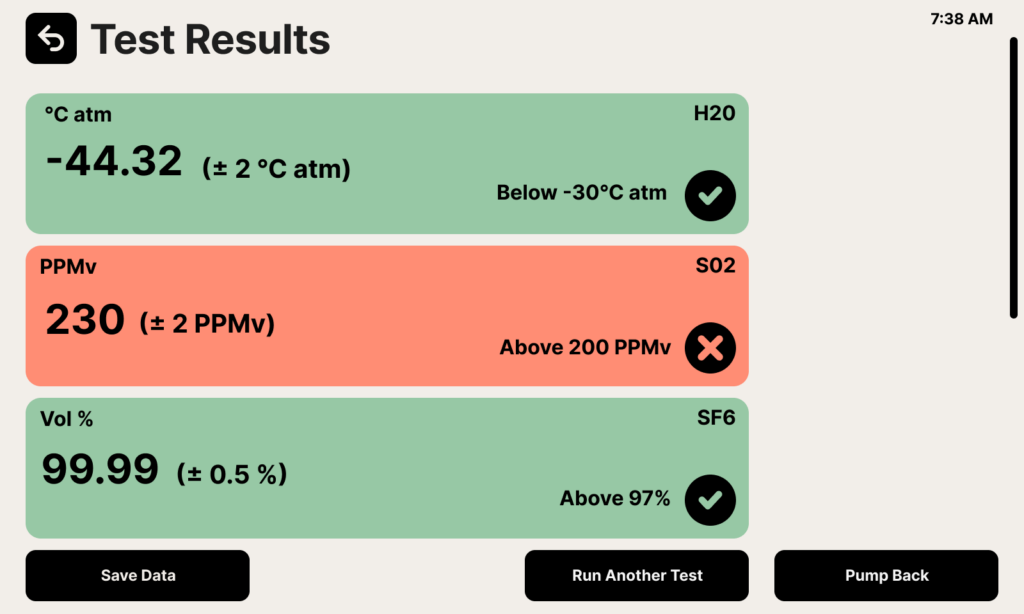
Understanding Sensor Tolerances
The accuracy of an SF6 analyzer’s measurements is affected by the tolerances of the individual sensors used to measure each parameter. Sensor tolerances refer to the degree of accuracy and precision that can be expected from the sensor in ideal laboratory conditions. In the field, conditions may be less than ideal, which can further affect the accuracy of the measurement.
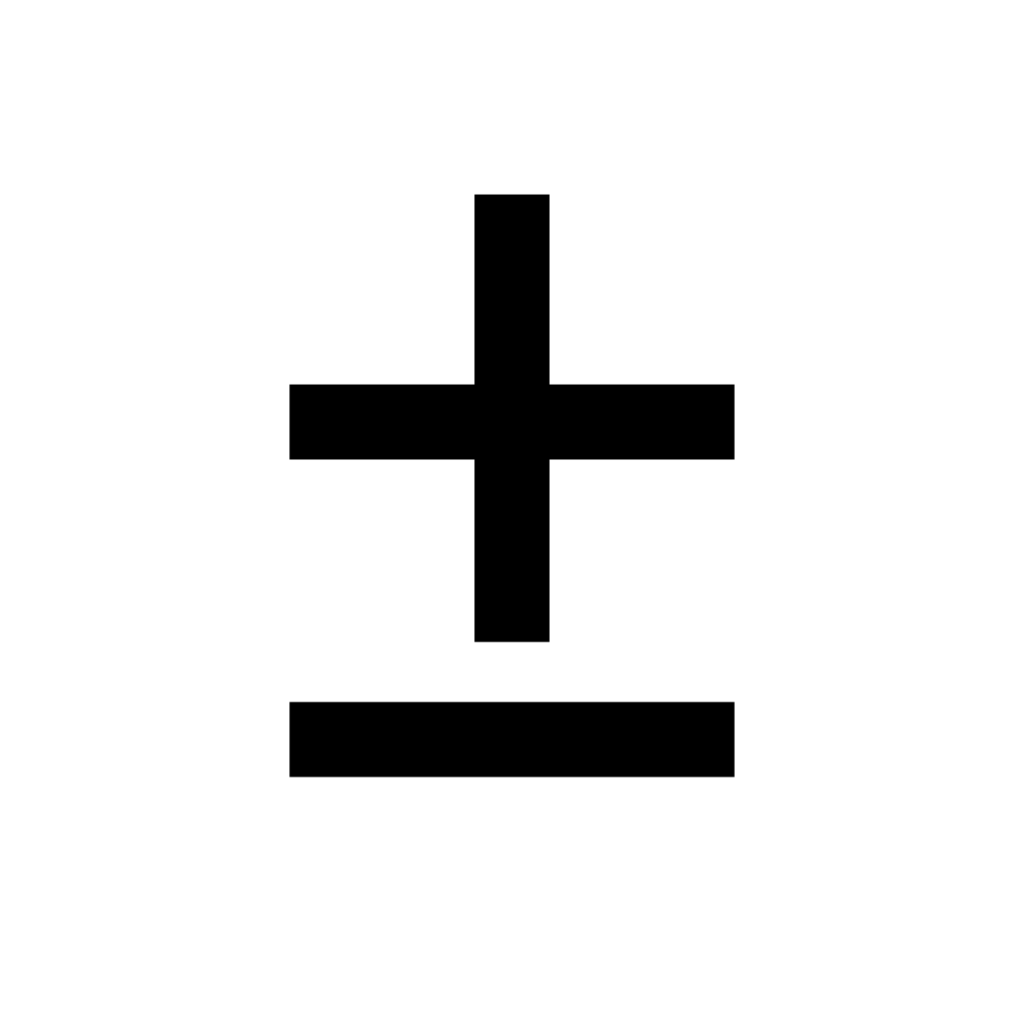
Here’s an example: The SF6 purity sensor might show a “97%” reading. The actual SF6 purity value may be as low as 95% or as high as 99%, given the tolerance range. Therefore, it is important to consider the acceptable range and the potential sources of error when interpreting the results of an SF6 purity test.
If you are experiencing some weird test results, try taking a few tests and averaging them to see where you fall. If you are having massive swings in measured values that are beyond what you expected given the sensor tolerances, try flushing your system with a bottle of nitrogen. If the device is still operating abnormally after that, it might need to be recalibrated or repaired.
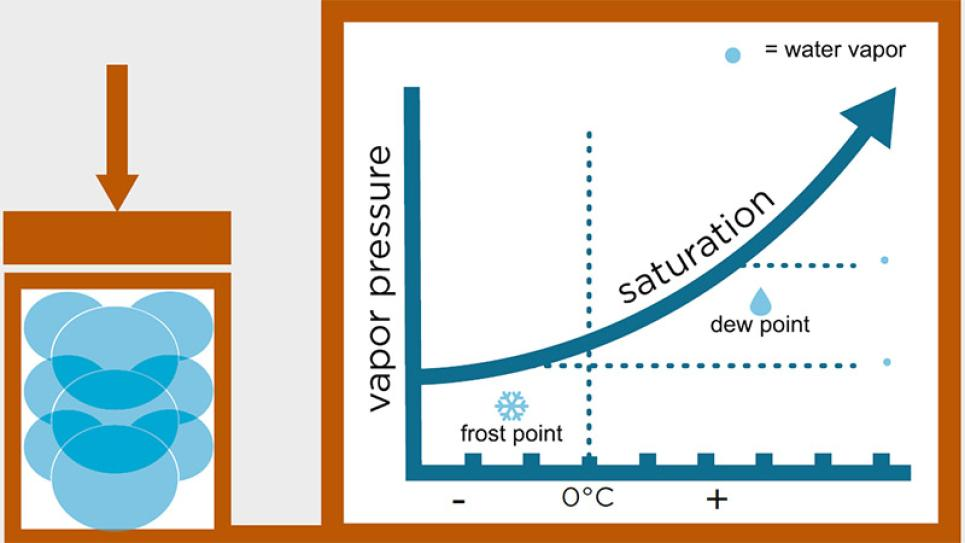
Dew Point vs. ppmv for Moisture
When it comes to measuring moisture in a gas, two common methods are used: parts per million by volume (ppmv) and dew point temperature. Both methods are used to express the amount of moisture in a gas, but they use different units and have different relationships.
PPMV is a unit of measurement that expresses the concentration of a gas in a mixture of gases. In the case of measuring moisture, ppmv expresses the amount of water vapor in a gas relative to the total amount of gases present in the mixture. For example, if a gas mixture contains 1 ppmv of water vapor, that means that for every 1 million molecules of gas in the mixture, 1 molecule is water vapor.
Dew point, on the other hand, is a measure of the temperature at which the water vapor in a gas will begin to condense into liquid water. It is expressed in degrees Celsius or Fahrenheit. When the temperature of a gas falls below the dew point, the water vapor will start to condense out of the gas and form liquid droplets.
The relationship between ppmv and dew point is influenced by factors such as temperature and pressure. Generally, as the dew point of a gas decreases, the ppmv of water vapor in the gas also decreases. This is because as the gas cools, it becomes less able to hold moisture, so the water vapor condenses out of the gas and the concentration of water vapor in the gas decreases.
Conversely, as the dew point of a gas increases, the ppmv of water vapor in the gas also increases. This is because as the gas gets warmer, it becomes more able to hold moisture, so the concentration of water vapor in the gas increases.
Therefore, to accurately measure the amount of moisture in a gas, it’s useful to consider both the ppmv of water vapor and the dew point temperature. By measuring both of these parameters, it is possible to obtain a more complete picture of the moisture content of the gas. Handy dew point calculator – https://dew-point.com/dew-point_calculator.asp
Verify Sensors are Calibrated & Accurate
To check the accuracy of an SF6 analyzer’s sensors, you can use calibrated gases with known certified gas concentrations. Here are the steps to follow:
- You will need to obtain certified gas mixtures of known SF6 concentrations from a reputable supplier. The supplier should provide a certificate of analysis that specifies the exact composition and concentration of the gas mixture. Pay attention to the tolerance that the supplier marks the certificate with.
- Connect the analyzer to a gas cylinder containing the certified gas mixture. Make sure that the gas flow rate is stable and set it to the recommended level.
- Allow the analyzer to stabilize for a few minutes to ensure that the readings have stabilized.
- Record the readings displayed on the analyzer for the known concentration of SF6 gas.
- Compare the readings obtained from the analyzer to the certified values of the SF6 concentration in the gas mixture. Calculate the percentage deviation from the certified value.